Non Invasive Glucose Meter Wins FDA Approval
Freestyle Libre ‘Flash’ Glucose Monitoring System.
The United States Food and Drug Administration (FDA) recently announced the long-awaited approval of Abbott Labs’ FreeStyle Libre Flash Glucose Monitoring System (Bloodless Glucose Meter). This is a meaningful development both for the company and for Diabetes patients, especially in the United States. However, it was expected. Abbott is already marketing this new noninvasive glucose meter and it was just a matter of time before the device received U.S. FDA approval.
When Can We Expect it in Major Retail Pharmacies?
Earlier this year, the company presented data from 50,000 Freestyle Libre users which show that patients are checking their blood sugar levels at least 16 times per day. This frequency is significantly higher compared to other monitoring systems. The high frequency of checking blood glucose levels is largely attributable to the fact that the FreeStyle Libre Flash Glucose Monitoring System does not require any finger pricking.
Since more frequent monitoring is usually associated with better health outcomes, particularly for diabetes patients, you can expect high demand for the device in the United States especially after winning FDA approval.
We expect the device to be launched in major pharmacies in the United States by the end of 2017. However, in the United States, FreeStyle Libre Flash is approved only for adults with diabetes. The FreeStyle Libre sensor can be considered a ‘replacement’ for fingersticks because it provides real-time readings and clear trends that are used for insulin dosing. Therefore, this device eliminates the need for any fingerstick calibrations.
In the United States, FreeStyle Libre Flash has three main differences:
- A shorter wear time (10 days).
- It will require a physician’s prescription.
- A significantly longer warm-up period after installation (12 hours).
Outside the United States, the device does not require a prescription, has a longer wear time of 14 days, and requires just a 1-hour warm-up.
One of the biggest changes is the longer war-up period which means that when you insert a new sensor, it will not provide any real-time blood glucose readings for the first 12 hours. Therefore, some users must go back to fingersticks during the warm-up period. Therefore, it’s recommended that you put a new sensor on before bed in order to get through much of the warm-up period. The other option is to buy two readers, overlap sensor wear times in order to avoid going for 12 hours without glucose data.
FreeStyle Libre Flash is a Continuous Glucose Monitor because it collects glucose data every minute and displays the reading and trend arrow. However, it’s essential to understand that it does not have an alarm and it does not communicate continuously with the reader device as other Continuous Glucose Monitoring (CGM) devices do.
The sensor must be scanned within 1.5 inches using the hand-held reader to get real-time glucose data. You can scan the sensor even through clothing making the process far less painful, visible and obtrusive than a fingerstick.
Read about the Top 5 Companies Developing Non-Invasive Glucose Monitors in 2022!

This approval comes one year after being submitted to the FDA for approval and a year since FreeStyle Libre Pro was launched in the US. This real-time version of FreeStyle Libre will expand the US Continuous Glucose Monitoring market significantly. The lower price, small on-body footprint, pharmacy distribution, no need for fingersticks, and ease of insertion are all very welcome improvements especially for diabetes patients.
FreeStyle Libre Flash is similar to convention CGM devices in that it measures the glucose levels in interstitial fluid in real time. However the device offers various advantages such as lower cost and longer sensor wear. Moreover in contrast to other CGM devices, FreeStyle Libre is factory calibrated meaning it doesn’t require fingerstick measurements for calibration.
New Information on Compatability with iPhone!
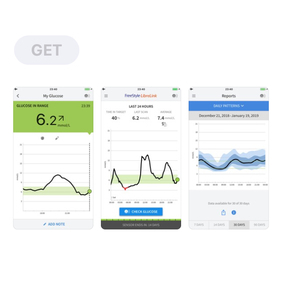
Features of the FreeStyle LibreLink App
Pros:
- Text-to-speech glucose readings. This is a very valuable feature for those who are visually impaired.
- The app is now available in more than 24 countries and supported in 26 languages.
- The option is available to make additional notes on insulin dosing, food, exercise and anything else relevant.
- The app has the ability to log small insulin doses (of 0.1 units)
Cons:
- You must have the app ‘open’ for it to scan – you can’t use it from the phone ‘lock’ screen
- persons ages 4-17 must be supervised by a caregiver 18years or older.
Most people are excited to see this version of FreeStyle Libre Glucose Monitoring System coming to the United States. Diabetes patients globally have responded positively to this device and it’s now being used by at least 400,000 people in more than 41 countries.


 These particular meters work by drawing interstitial fluid through the sweat glands in the skin by using a small electric current. This electric current is so small that the only real discomfort associated with using the device is some possible irritation caused by wearing the device around the wrist or arm, and this is usually no more uncomfortable than wearing a large watch or MP3 player. The
These particular meters work by drawing interstitial fluid through the sweat glands in the skin by using a small electric current. This electric current is so small that the only real discomfort associated with using the device is some possible irritation caused by wearing the device around the wrist or arm, and this is usually no more uncomfortable than wearing a large watch or MP3 player. The 
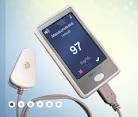 Although there are various technologies being used to develop non invasive testing devices including florescent technology, electromagnetic sensing, mid-infrared spectroscopy and ultrasound technology among other technologies, not all have received FDA approval yet. However, the GlucoTrack has already received the CE Mark approval for use in the EU (European Union).
Although there are various technologies being used to develop non invasive testing devices including florescent technology, electromagnetic sensing, mid-infrared spectroscopy and ultrasound technology among other technologies, not all have received FDA approval yet. However, the GlucoTrack has already received the CE Mark approval for use in the EU (European Union).